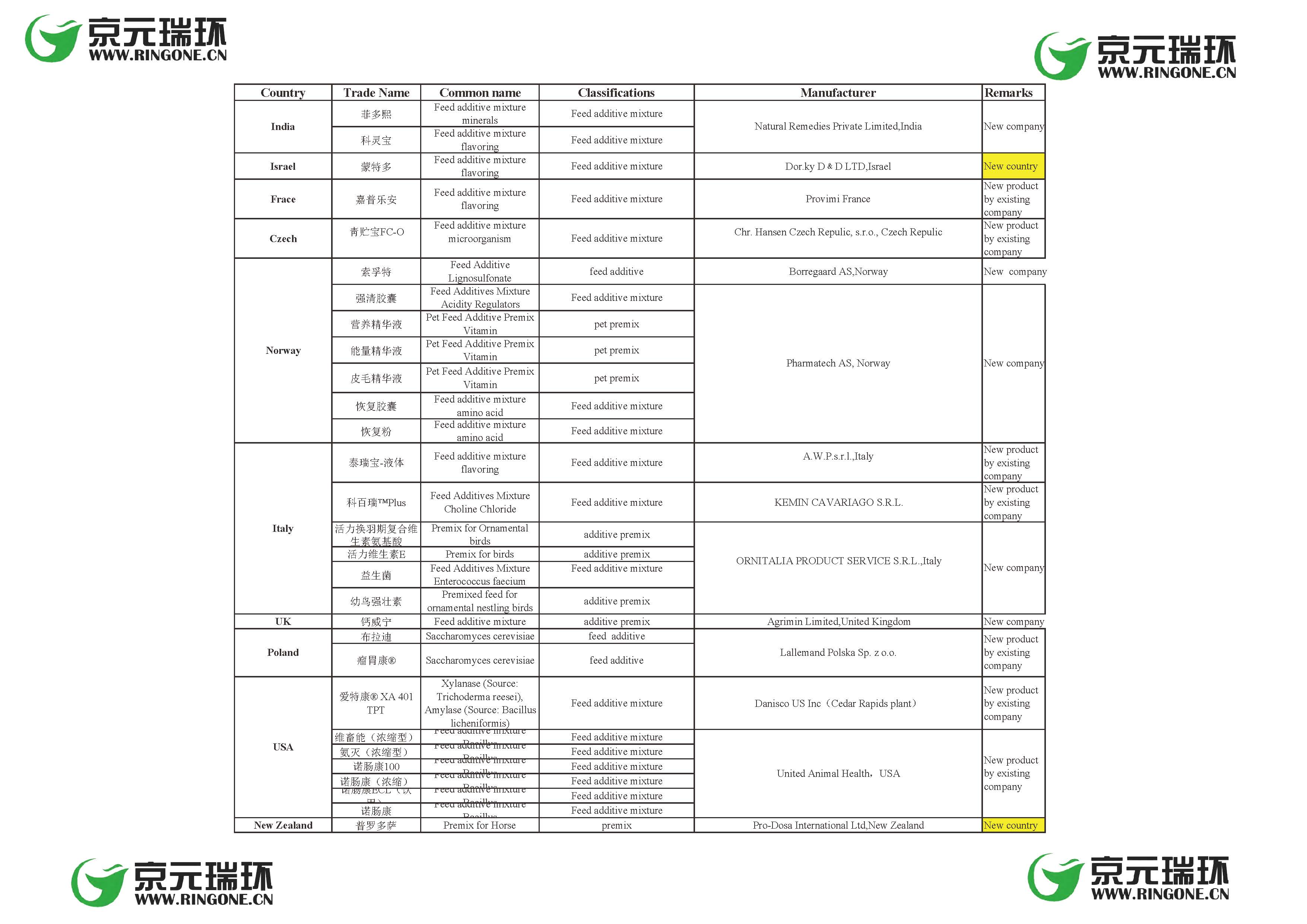
General Administration of China customs updates the “GACC approved List of countries (regions) for import feed additives and premix” on April 28th, with 29 additions of new feed additive products, and 2 new country additions (New Zealand and Israel). New additions involves 14 foreign feed additive production companies:
Natural Remedies Private Limited,India
Dor.ky D﹠D LTD,Israel
Provimi France
Chr. Hansen Czech Repulic, s.r.o., Czech Repulic
Borregaard AS,Norway
Pharmatech AS, Norway
A.W.P.s.r.l.,Italy
KEMIN CAVARIAGO S.R.L.
ORNITALIA PRODUCT SERVICE S.R.L.,Italy
Agrimin Limited,United Kingdom
Lallemand Polska Sp. z o.o.
Danisco US Inc(Cedar Rapids plant)
United Animal Health,USA
Pro-Dosa International Ltd,New Zealand
The update does not necessarily mean that they will be able to export to China immediately. In pursuant of Artile 3, “Measures for the Administration of registration of imported feed and feed additives” by Ministry of Agriculture and Rural Affairs of P.R. China, “The overseas enterprise shall file an application for import registration with the Ministry of Agriculture and obtain an import registration license for feed and feed additives before exporting a feed and feed additive to China for the first time.” Imported feed and feed additives shall not be sold and used in China without an import registration license. As a result, those companies listed in GACC approved list should also complete MARA product registration. The export can be eligible when both GACC and MARA registration competed. Remind to importers: please confirm with foreign feed additive suppliers if they have product registrations from MARA before signing contract. Or importers may encounter custom clearance problems during import link.
